Can Pharmaceutical Companies Choose Ink Jet Printers Or Laser Printers To Better Meet Gmp Regulatory Requirements?
Can Pharmaceutical Companies Choose Ink Jet Printers Or Laser Printers To Better Meet Gmp Regulatory Requirements?
Boxes are a common method of packaging in the pharmaceutical industry, and three phase codes are printed on the medicine boxes: production date, product batch number, and expiration date, which are mandatory requirements of the country. What are the application characteristics of inkjet printers in the pharmaceutical industry? What are the changes in the requirements of pharmaceutical companies for inkjet labeling? Chengdu Linservice believes that the comprehensive implementation of GMP certification in the pharmaceutical industry requires very strict standards for various equipment and packaging materials. The packaging of drugs requires high speed and cleanliness. At the same time, the shapes of pharmaceutical packaging bottles are diverse, requiring the use of specialized material handling systems tightly integrated with printing and labeling equipment. In addition, in the downstream stage of the inkjet process, it is necessary to high-pressure sterilize the medicine bottle, which requires good adhesion of the label, and relevant environmental certification for inkjet printing ink, solvents, etc. Chengdu Linservice has served pharmaceutical enterprises for nearly 20 years. It is understood that the application of pharmaceutical enterprise supervision code inkjet printing in paper, plastic, metal, glass, ceramics, Coated paper, thermal paper, coated paper, Aluminium foil, uncoated paper, ABS, PET, PVC, PE, Tinning, gold foil, plastic packaging and other surface automatic inkjet printing databases, date, time, batch number, shift, serial number, etc. are far more diversified than the needs of some food enterprises. Faced with various industry application characteristics, Chengdu Linservice has also put a lot of effort into the selection of inkjet printers and the allocation of consumables. From research and development, production to intelligent manufacturing, targeted optimization and transformation have been carried out according to industry characteristics, achieving more environmentally friendly labeling, more efficient on-site applications, higher automation level compared to similar products, and stronger functional configuration.
What are the general solutions for inkjet printers in terms of drug box packaging? In terms of drug box packaging, Chengdu Linservice provides a series of automated solutions for high-speed production lines and small products, including food grade ink that can be directly labeled on capsules and tablets. Chengdu Linservice’s micro character inkjet printer can perform non-contact labeling operations and print content as small as 0.8mm in height, meeting the reality of more small drug sizes and limited labeling content space, making it an ideal partner for major pharmaceutical manufacturers.
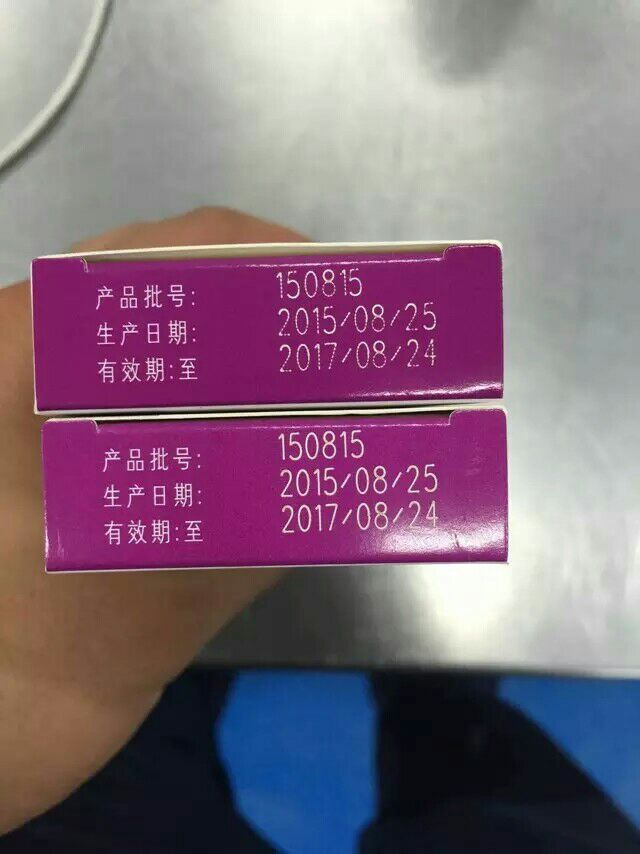
After completing the common applications of inkjet printers, the editor of Chengdu Linservice will tell you about two common types of inkjet printer selection: one is inkjet printers such as small character inkjet printers and high-resolution inkjet printers; one type is a laser marking printer with stronger anti-counterfeiting and traceability capabilities. Is the customer choosing an ink jet printer or a laser printer? The most commonly used small character machines are still small size drug packaging boxes, which usually print date, batch number, specifications, and other information, such as the EC1000 inkjet printer of Chengdu Linservice; the high-resolution inkjet printer can print higher resolution identification content, meeting the requirements of pharmaceutical manufacturers with certain standards for DPI, such as Chengdu Linservice's LS-X100 thermal foaming inkjet printer. The M7031 laser printer from Chengdu Linservice belongs to a more environmentally friendly and green labeling application. The initial procurement cost will be relatively high, but the later use cost will be very low and the failure rate will be low. The marked content has anti-tampering, anti-counterfeiting and other functions, which is very suitable for some pharmaceutical enterprises with strict environmental requirements. The production coding application of these two drugs in the pharmaceutical industry meets the constantly increasing and differentiated customer needs:
1. Faster speed requirement for inkjet printing. In today's increasingly automated production lines, many pharmaceutical companies have been equipped with more advanced processes and assembly lines, with significant improvements in speed. Some traditional inkjet printer equipment can no longer meet the labeling requirements. The EC1000 series of Chengdu Linservice has made great progress in speed after deep optimization and research, achieving a high-speed inkjet speed of 360m/min, which can meet the inkjet speed requirements of most pharmaceutical packaging.
2. Stability requirements for inkjet equipment. An inkjet printer is applied to industrial grade drug production lines, and its stability and intelligence are equally important. It is a core indicator that manufacturers need to focus on. A stable inkjet printer can maintain long-term high-speed operation and long service life; a poorly stable inkjet equipment often experiences unexpected situations such as ink leakage and malfunctions, which can seriously affect the production line's output and production capacity, and directly affect the company's efficiency. Chengdu Linservice's EC series small character inkjet printer and LS series laser printer have both received time verification from customers on site.
3. The environmental requirements for labeling are mainly reflected in the impact of consumer on product packaging, whether ink has undergone formal ROSH testing to meet the production needs of green and environmental protection, and whether it will have adverse effects on the drug itself, which are important factors that need to be calculated.
The importance of labeling work in the pharmaceutical industry is self-evident. In the future, Chengdu Linservice not only needs to meet the simple labeling problems of pharmaceutical production enterprises, but also involves an overall automated labeling traceability marketing solution, providing the pharmaceutical industry with "one item, one code" labeling services, controlling the quality of drugs from the source, and doing a good job in front-end production work. In the back-end consumer end, it will also introduce and collect information on drugs, assist enterprises in making decisions. For more requirements, please contact Chengdu Linservice. Welcome to call: +86 13540126587.


 English
English Español
Español Português
Português русский
русский français
français 日本語
日本語 Deutsch
Deutsch Tiếng Việt
Tiếng Việt Italiano
Italiano Nederlands
Nederlands ไทย
ไทย Polski
Polski 한국어
한국어 Svenska
Svenska magyar
magyar Malay
Malay বাংলা
বাংলা Dansk
Dansk Suomi
Suomi हिन्दी
हिन्दी Pilipino
Pilipino Türk
Türk Gaeilge
Gaeilge عربى
عربى Indonesia
Indonesia norsk
norsk اردو
اردو čeština
čeština Ελληνικά
Ελληνικά Українська
Українська Javanese
Javanese فارسی
فارسی தமிழ்
தமிழ் తెలుగు
తెలుగు नेपाली
नेपाली Burmese
Burmese български
български ລາວ
ລາວ Latine
Latine Қазақ
Қазақ Euskal
Euskal Azərbaycan
Azərbaycan slovenský
slovenský Македонски
Македонски Lietuvos
Lietuvos Eesti Keel
Eesti Keel Română
Română Slovenski
Slovenski मराठी
मराठी Српски
Српски 简体中文
简体中文 Esperanto
Esperanto Afrikaans
Afrikaans Català
Català עִברִית
עִברִית Cymraeg
Cymraeg Galego
Galego Latvietis
Latvietis icelandic
icelandic יידיש
יידיש Беларус
Беларус Hrvatski
Hrvatski Kreyòl ayisyen
Kreyòl ayisyen Shqiptar
Shqiptar Malti
Malti lugha ya Kiswahili
lugha ya Kiswahili አማርኛ
አማርኛ Bosanski
Bosanski Frysk
Frysk ជនជាតិខ្មែរ
ជនជាតិខ្មែរ ქართული
ქართული ગુજરાતી
ગુજરાતી Hausa
Hausa Кыргыз тили
Кыргыз тили ಕನ್ನಡ
ಕನ್ನಡ Corsa
Corsa Kurdî
Kurdî മലയാളം
മലയാളം Maori
Maori Монгол хэл
Монгол хэл Hmong
Hmong IsiXhosa
IsiXhosa Zulu
Zulu Punjabi
Punjabi پښتو
پښتو Chichewa
Chichewa Samoa
Samoa Sesotho
Sesotho සිංහල
සිංහල Gàidhlig
Gàidhlig Cebuano
Cebuano Somali
Somali Точик
Точик O'zbek
O'zbek Hawaiian
Hawaiian سنڌي
سنڌي Shinra
Shinra հայերեն
հայերեն Igbo
Igbo Sundanese
Sundanese Lëtzebuergesch
Lëtzebuergesch Malagasy
Malagasy Yoruba
Yoruba Javanese
Javanese Divih
Divih Philippine
Philippine Gwadani
Gwadani Kurde
Kurde



What is Online Inkjet Printer?
With the rapid development of modern printing technology, Online Inkjet Printer, as an efficient and accurate printing device, is being adopted by more and more enterprises and industries. It is not only suitable for ordinary printing tasks, but also plays an important role in many occasions that require high-quality and fast output. So, what is an online inkjet printer? What are its unique advantages and applications?
Read MoreUV Handheld Inkjet Printer: The Future of Portable Printing Solutions
In today’s fast-paced industrial and commercial environments, the demand for efficient, high-quality, and portable printing solutions is at an all-time high. The UV Handheld Inkjet Printer is quickly gaining popularity as a versatile tool for a wide range of applications, offering businesses a mobile, on-the-spot printing solution with unparalleled flexibility and precision.
Read MoreWhat is the characteristic of inkjet printer
Inkjet printers have become a popular choice in both home and office settings due to their unique characteristics.
Read More